Notes on Atomic Structure
Bohr's Atomic Model
En
= -KZ2 / n2 where K = 13.6 ev, Z = atomic number and n = number of orbit from the nucleus.
rn = a0 n2 / Z , where a0 = Bohr’s first orbit radius = 0.529 A0
ΔE = Ef - Ei , where Ef = energy of
final orbit and Ei = energy of initial orbit
Also
when an electron jumps from a higher orbit to the lower orbit, then difference
in energy (Ef - Ei) is emitted.
8. Only those orbit are
allowed for the electrons to revolve round the nucleus, for which the angular
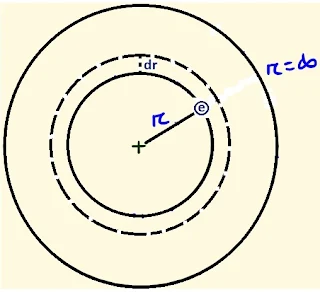
momentum (mvr) of the electron is a whole number multiple of h/2π. Mathematically, mvr = nh/2π. This principle is called quantisation of angular
momentum.
=> v2
= Ze2/4πε0 mr .......... eq. 1
since
mvr = nh /2π
=> v
= nh /2πmr
=> v2
= n2h2 /4π2m2r2
…… eq.2
From eq 1 and 2,
n2h2
/4π2m2r2 =
Ze2/4πε0 mr
=>
r = n2h2 ε0 / πme2z
=> r
= (h2 ε0 / πme2) (n2/Z)
=> r = a0 (n2/Z) where a0 = (h2 ε0 / πme2) = Bohr’s first orbit radius for hydrogen atom
Putting
the value of constants, we get a0 = 0.529 A0 = 0.0529 nm
For
hydrogen atom, Z = 1
If
n =1, r1 = a0 = 0.529 A0
If
n =2, r2 = 4a0 = 4 X 0.529 A0
CLICK ME -GET ALL CHEMISTRY NOTES
Problem 1: Determine the second Bohr's orbit of He+ ion.
Solution: The atomic number of He+ ion = z = 2
=>r2 = a0 n2 / Z = 0.529 (22 / 4) = 2 X 0.529 A0
Solution: r = a0 (n2/Z)
r(H) = a0 (n2/Z) = a0 n2
r (He+) = a0 (n2/Z) = a0 (n2/2)
r(H)/r(He+) = 2
=> r(H) = 2 r(He+)
We know mvr = nh / 2π
Putting the value of r = a0 n2 / Z
Vn = velocity of Bohr's nth orbit = 2.18X106 (z/n)m s-1
Solution: We have Vn = 2.18X106 (z/n)m s-1
For He+ ion, z = 2, Second Bohr's orbit => n = 2
=> Vn = 2.18X106 m s-1
Note: The formula of velocity of electron in nth Bohr's orbit indicates that, all hydrogen like species having same z and n will have velocity equal to 2.18X106 m s-1
Assignment: Determine the Bohr's orbit of Li2+ ion in which the electron moves at a speed equal to the speed of electron in the first Bohr's orbit of hydrogen.
The number of revolutions per second (RPS) ∝ Velocity of electron (V)
The number of revolutions per second (RPS) ∝ 1/circumference of orbit (2πr)
=> The number of revolutions per second (RPS) = V/2πr
Putting the value of V = nh/2πmr
RPS = (nh / 4π2mr2)
and r = a0 n2 / Z
=> RPS = (nh/4 π2m) (z2/ a0 n2)2
=> RPS = (h/4π2m a02) (z2 /n3)
Putting the values of the constants,
=> RPS = 6.584X1015 (z2 /n3) revolutions /s
Since mv2 /r = Ze2/4πε0 r2
½ (mv2) = Ze2/8πε0 r = K.E
Potential energy of the electron is the energy possessed by it due to its position w.r.t. the nucleus. Consider an electron present at infinite distance apart from the nucleus, where its energy is considered to be zero. As it comes closer to the nucleus (into the influence of nucleus) it looses energy, hence its energy becomes negative when it is placed in any orbit.
Its now clear from the above logic that the potential energy of an electron in a given Bohr's orbit is the negative of the work done required to move that electron from the said Bohr's orbit to the infinity Bohr's orbit.
An infinitesimal work done by an infinitesimal displacement of the electron is given by, dW = F dr
Thus the total work done in displacing the electron to the infinity orbit should be,
= (Ze2/8πε0 r) + (- Ze2/4πε0 r) = - Ze2/8πε0 r
E (Total) = - Z2e2/8πε0 n2 = (- e2/8πε0)( Z2/n2)
En =Energy of nth Bohr's orbit = - K (Z2/n2)
Problem: Calculate the energy of electron in the Bohr's first orbit of hydrogen atom in kilo Joule per mole.
E1= - K (Z2/n2) = - 2.18 X 10-18 Joule / atom = - 2.18 X 10-18 J X 6.02 X 1023 Joule/ mole
= - 2.18 X 10-18 J X 6.02 X 1023 x 10-3 K Joule / mole = - 1312 K Joule / mole
Transition Energy and Ionisation Energy: When ever an electron jumps from any initial orbit (ni) to the final one (nf), energy is absorbed or lost depending on whether it jumps up or down respectively.
Mathematically, the energy absorbed or lost = ΔE = Ef - Ei
=> ΔE = - K (Z2/nf2) - {- K (Z2/ni2)} = KZ2 {(1/ni2)
- (1/nf 2)}
Thus ionisation energy,
ΔE = KZ2 {(1/ni2)
- (1/nf2)}
= KZ2 {(1/ni2)
- (1/∞2)}
= KZ2/ni2
Problem: Calculate the energy required to excite an electron of hydrogen atom from first Bohr's orbit to the third orbit and also calculate the amount of energy emitted when the same electron jumps down from that 3rd Bohr's orbit to the first one.
For hydrogen atom, Z = 1, when electron jumps up from first to the third orbit,
ni = 1 and nf = 3.
Thus ΔE = K {(1/12) - (1/32)}
= 8/9 K
= 8/9 X 13.6 eV amount of
energy is gained.
When the electron jumps down from third Bohr's orbit to the first one in hydrogen atom, ni = 3 and nf = 1.
Thus ΔE = K {(1/32)
- (1/12)}
= - 8/9 K
= -8/9 X 13.6 eV
That means the same
amount of energy is released.
Problem: Determine the third ionisation energy of lithium atom.
Z = 3, ni = 1, ΔE = KZ2/ni2
Emission Spectrum of Hydrogen atom:
Electrons of hydrogen atoms when absorb energy, get excited to the next higher shells. These excited electrons emit energy (in form of light), when they jump down to the lower energy levels. These energy (emitted light) are observed on a fluorescent screen which appear as group of lines. The number of lines observed on the screen indicates the number of possible transitions made by excited electrons.
ΔE = KZ2 {(1/ni2)
- (1/nf2)}
=> ΔE = hν =
hc/λ = KZ2 {(1/ni2)
- (1/nf2)}
=> 1/λ = ṽ = (KZ2/hc) {(1/ni2)
- (1/nf2)}
=> ṽ = RH Z2 {(1/ni2) - (1/nf2)} ,
Where RH = K/hc = Rydberg's constant = 1.0973 X 107 m-1
1/λ = ṽ = wave number
In Paschen series, ni = 3 and nf = 4, 5, 6,
.... This
series falls in the infrared region of the electromagnetic spectrum.
In Brackett series, ni = 4 and nf = 5, 6, 7,
.... This
series falls in the infrared region.
In Pfund series, ni = 5 and nf = 6, 7,
8, .... This
series falls also in the infrared region.
=> 1/λ = ṽ =
wave number
= RH Z2 {(1/ni2) - (1/nf2)} ,
Since C = νλ => ν = frequency
= C/λ = C . ṽ
= C . RH Z2 {(1/ni2) - (1/nf2)}
put value for ni and nf
C = 3 X 108 m/sec
RH = 1.0973 X 107 m-1
and then get answer.
Correctness or Validity or Advantages of Bohr's Atomic Model:
Disadvantages or Drawbacks or Limitations of Bohr's Atomic model:
Sommerfeld's Atomic Model
Sommerfeld’s atomic model is an
improvement over Bohr’s atomic model. It was Introduced by Arnold Sommerfeld in
1916, which refined Bohr's theory by incorporating the concept of elliptical
orbits and relativistic corrections. While Bohr focused on the circular path
called orbit or Shell, Sommerfeld introduced a new type of elliptical path for
electrons to explain the splitting of fine lines obtained in Hydrogen spectrum.
His theory helped to understand how subshells make a shell.
Postulates of Sommerfeld’s Atomic Model
Sommerfeld refined Bohr’s atomic
model by introducing elliptical orbits and relativistic corrections. His
postulates are:
1. Electrons Move in Discrete Orbits
Similar to Bohr’s model, Sommerfeld
stated that electrons revolve around the nucleus in definite orbits without
radiating energy.
2.Azimuthal Quantum Number and Sub-Shells
Sommerfeld used the letter k for Azimuthal quantum number but later the letter l was used. He suggested that, for any principal quantum number n, the values of azimuthal quantum number k vary from 1 to n. Each value of the k for any particular value of n represents a different subshell (an eliptical path) under the partuclar shell.
For example, if n=3, then different values of k will be equal to 1 to 3. i.e., 1 and 2 and 3. Thus threee different values of k represent diffrent subshells (eliptical paths) for the electrons. Different possibilities are n=3, k=3 and n=3, k=2 and n=3,k=1
This explained the splitting of energy levels into sub-levels (s, p, d and so on).
3.Introduction of Elliptical Orbits
Unlike Bohr, who proposed circular orbits, Sommerfeld suggested that electrons move in elliptical orbits with the nucleus at one of the foci.
Circular orbits are a special case of elliptical orbits when the eccentricity is zero. We can futher draw a figure to summerise the postulates 2 and 3.
Since electrons moving in inner
orbits have high velocity, Sommerfeld applied Einstein’s theory of relativity
to correct Bohr’s model.
The faster an electron moves, the
greater its mass, leading to slight energy level variations and explaining the
fine structure of spectral lines.
7. Fine Structure of Spectral Lines
Sommerfeld’s model successfully
explained the fine structure (small splitting) in the hydrogen spectrum, which
Bohr’s model failed to do.
8.Zeeman Effect and Stark Effect (Partial Explanation)
Sommerfeld’s model attempted to
explain the Zeeman effect (splitting of spectral lines in a magnetic field) and
Stark effect (splitting in an electric field), though it was not completely
successful.
Drawbacks of Sommerfeld's Atomic Model
1.Failure to Explain Spectral Lines of
Multi-Electron Atoms
While Sommerfeld’s model improved
upon Bohr’s by explaining fine structure in hydrogen, it could not accurately
explain the spectra of atoms with more than one electron.
2.Violation of Uncertainty Principle
The concept of well-defined
elliptical orbits contradicts Heisenberg's Uncertainty Principle, which states
that the exact position and momentum of an electron cannot be simultaneously
determined.
3.No Justification for Elliptical
Orbits
The assumption of elliptical orbits
was introduced to fit experimental data rather than derived from first
principles, making the model less theoretically sound.
4.Inconsistency with Electron Spin
Sommerfeld’s model did not account
for electron spin, which was later introduced by Goudsmit and Uhlenbeck to
explain additional spectral features.
Conclusion
Sommerfeld’s atomic model was an important step toward modern quantum mechanics. It introduced elliptical orbits, quantum numbers, and relativistic effects, paving the way for Schrödinger’s wave mechanical model of the atom.
Electromagnetic Wave
An electromagnetic (EM) wave is a
type of wave that consists of oscillating electric and magnetic fields, which
propagate through space at the speed of light. These waves do not require a
medium and can travel through a vacuum.
Key Characteristics of
Electromagnetic Waves:
Perpendicular Fields:
The electric field (E) and the
magnetic field (B) oscillate perpendicular to each other.
Both fields are also perpendicular to
the direction of wave propagation, making it a transverse wave.
Speed of Light:
In a vacuum, all EM waves travel at
the speed of light (ccc) which is approximately 3.0×108m/s.
Self-Propagating:
A changing electric field generates a
magnetic field, and a changing magnetic field generates an electric field. This
continuous interaction allows EM waves to propagate.
Energy Transmission:
EM waves carry energy and momentum
and can exert pressure, known as radiation pressure.
Electromagnetic Spectrum:
EM waves exist in a broad range of
wavelengths and frequencies, forming the electromagnetic spectrum, which
includes:
Radio waves (used in communication)
Microwaves (used in cooking and
radar)
Infrared waves (felt as heat)
Visible light (what we see)
Ultraviolet (UV) rays (causes
sunburn)
X-rays (used in medical imaging)
Gamma rays (emitted by radioactive
materials and used in cancer treatment)
Mathematical Representation:
The relation between frequency, speed and wavelength of an EM wave is:
C = f . λ
An electromagnetic wave traveling in
the x-direction can be described by:
E=E0sin(kx−ωt)
B=B0sin(kx−ωt)
where:
E0, B0= maximum
electric and magnetic field amplitudes
k = wave number (k=2π/λ )
ω = angular frequency (ω=2πf)
f = frequency
λ = wavelength
Problem: Calculate the wavelength of red light having frequency 4.27 X 1014 Hz.
Solution: C = f . λ
=> λ = C/f
=> λ = (3 X 108 m/sec) / (4.27 X 1014)
= 8.9 X10-7 m = 890 10-9
m
= 890 nm
Planck's Quantum Theory:
Statement: Energy
emitted or absorbed by a body is not continuous but discontinuous in form of
discrete energy packets. Each discrete energy packet is called a quantum (or called
photon in case of light energy). Energy of a quantum is directly proportional
to its frequency and the total energy absorbed or emitted by a body per second
is equal to the product of energy of a quantum and the number of quanta absorbed
or emitted by a body per second.
Mathematically,
Energy of a quantum, E ∝ f
=> E = h.f,
where h = Planck’s constant
h= 6.625 X 1034 joule sec.
and f = frequency
of each quantum
And the total energy absorbed or emitted by a body per second, E = n.h.f,
where n = number of quanta absorbed or emitted per second.
Problem: A discharge lamp rated with power of 25 Watt emits yellow light of wavelength 580 nm. How many photons of yellow light does the lamp generate in one second?
Solution:
Energy = Power X time
= 25 x 1 = 25 joule
Since, E = n.h.f
n = no. of photons = E/(h.f)
=>n = E/[h(C/ λ)] (Since C =f λ)
=>n = (25 j X 5.8 X 10-7)/
(6.625 X 1034
X 3 X 108)
de Broglie Concept of Dual Nature of Matter:
Statement: All forms of matter including
electrons, protons or neutrons show dual nature. i.e., wave nature and particle
nature.
Derivation:
From Planck’s quantum theory,
E = h.f = hc/λ -------1
From
Einstein’s equation,
E
= mC2 --------- 2
From
equation 1 and 2,
mC2
= hc/λ
=> λ = h/mc = h/p
------- 3
Where
p = momentum of the particle.
or
p = mass X velocity
Equation
3 is called de Broglie equation
de broglie wavelength of an electron accelerated by a potential difference of v - volts:
If
an electron at rest is accelerated by a potential difference of V volts, its
kinetic energy,
(1/2)
mv2 = e.V (since
electrical work done = charge X voltage)
(Note
that v stands for velocity whereas V stands for potential)
=>
v = {(2eV)/m}1/2
Substituting
for velocity in equation 3,
=>
λ = h/m {(2ev)/m}1/2
=> λ = h/(2meV)1/2 --------- 4
In the above equation when we put the value of 'h'(Planck's constant), mass and charge of electron and vary the value of Voltage 'V' between 10 to 10,000 volts, we get λ varying between 1.226 A0 to 3.977 A0 which coincides with the order of X-rays. This has been confirmed experimentally by Davision and Germer. The diffraction pattern obtained from the electron beam (produced from a tungsten filament) was similar to that of X-ray diffraction. Thus electrons exhibit wave character.
When electrons strike on ZnS screen they create spot of light which do not spread to the surrounding, area confirming its particle character.
Validity of Bohr's Theory Confirmed by de-Broglie equation:
According to Bohr, mvr = nh/2 π
=>2.π.r = nh/mv= nλ
(Since λ = h/mv)
The
above equation shows that integral number ‘n’ of wavelength ‘λ’ can be
accommodated in Bohr’s orbit having circumference 2πr. In other words, there will be always a whole
number of waves in an orbit. We can say the electron waves are in phase.
But when 2πr is not equal
to whole number multiple of λ, we say the electron waves are out of phase.
Thus
de-Broglie justified Bohr’s angular momentum equation.
Mass of 1 helim atom = 4/6.02X1023
g
=6.64X10-27kg
Putting
all the values we can get the answer.
Problem: An electron diffraction experiment was performed using electron beam accelerated by a potential difference of 10kV. Calculate the wavelength of the electron beam.
Solution: λ = h/(2meV)1/2
Put the value of h, mass and charge of electron and V = 10 X 103 = 104 volts, and get the answer.
Heisenberg’s Uncertainty Principle:
Heisenberg's Uncertainty Principle is
one of the fundamental ideas in quantum mechanics, proposed by Werner
Heisenberg in 1927. It expresses a fundamental limit to the accuracy with which
certain pairs of physical properties (called
complementary variables) of a particle can be known simultaneously.
Statement using position and momentum as complementary variables:
It is impossible to measure
simultaneously both the position (x) and momentum (p) of a microscopic particle
with absolute accuracy and certainty.
Mathematically,
Δx⋅Δp ≥ h/4π
Or
Δx⋅mΔv ≥ h/4π
Where = Uncertainty in position and
Δp = Uncertainty in momentum (momentum = mass × velocity), Δv = Uncertainty in
velocity
h = Planck’s constant ≈ 6.626×10−34 Js
This inequality means that the more precisely we know a particle’s
position, the less precisely we can know its momentum, and vice versa.
For example:
If a particle is localized very
tightly (i.e., we know where it is), its wavefunction is sharply peaked. This
means its momentum spectrum is spread out—so its momentum is uncertain.
If a particle has a well-defined
momentum (narrow momentum distribution), its position is spread out means it’s
not localized.
Other Uncertainty Pairs
The principle isn’t just limited to
position and momentum. It applies to other pairs of complementary or conjugate
variables, such as:
Energy (E) and Time (t)
Angular momentum (Lz) and Angular position (φ)
For energy and time the mathematical
expression will be,
ΔE⋅Δt ≥ h/4π
If the time for which a system
remains in a particular energy state is long then its energy will not be well
defined. On the other hand, if the time for which the system remains in a
particular energy state is short then its energy will bfe more defined.
Limits of Measurement: It’s
impossible to design an experiment that simultaneously measures the position
and momentum with arbitrary precision.
Example: Electron in an Atom
In an atom, electrons are bound to
the nucleus. If you try to localize an electron too closely (small Δx), you
need light radiation of shorter wavelength (high energy). When such a radiation
strikes an electron, some of the energy is transferred to the electron and the
velocity or momentum of the electron changes. Similarly if light of longer wavelength is
used then electron can’t be located with accuracy whereas there will be no
change in velocity or momentum.
Significance of uncertainty principle:
1. If we can't determine the exact position of an electron with accuracy then how can we think of a definite path of an electron. For this reason the idea of definite path for an electron has been discarded by the uncertainty priciple.
2. uncwertainty principle is npot applicable to to macroscopic bodies as photons can't alter their position or momentum.
3. By taking proper substitution in the formula, we can show that electron can't exist inside nucleus.
Problem: Calculate the uncertainty in position of a tiny particle with mass equal to 1mg if its uncertainty in its velocity is 5.5X10-20 m/s.
Solution:
m =1mg = 10-3 kg, Δv = 5.5X10-20 m/s, π = 3.14, Δx = ?
Δx⋅mΔv ≥ h/4π
Put the values and get the answer.
Problem 2: On the basis of Heisenberg's uncertainty principle, show that an electron can not exist inside nucleus. (Radius of nucleus is of the order 10-15m, mass of electron is = 9.1 X 10-31 kg, h = 6.626×10−34 Js)
Solution: Δx⋅mΔv ≥ h/4π
=> Δv = h/4π.m.Δx
putting all the values we get,
Δv = 5.77x1010 m/s which is greater than velocity of light which is impossible. Hence electron can’t exist inside nucleus.
Note: 1. If the uncertainty in position is given within ± 1nm, then Δx should be taken (in the range) as 2 nm.
2. If the uncertainty in position given is of the order ± 1nm, then Δx should be taken as 1nm.
3. If the uncertainty in velocity is given within ± 1m/s, then Δv should be taken (in the range) as 2 m/s.
4. But if the uncertainty in velocity is given as 500 ± 1m/s, then Δv should be taken as ± 1m/s.
Schrodinger’s Wave Equation:
Schrodinger developed a mathematical differential equation whose solution could give the electron distribution around nucleus as well as the allowed energy levels of a particle (electron) in a given field.
Let us consider an electron wave moving along x- axis and behaving like a standing wave. The amplitude function for this wave can be written as:
Watch this Lecture Video for the derivation of Schrodinger Wave Equation
Quantum Numbers:
The idea of shell was made from Bohr’s atomic model and the idea of Subshells
under a Shell was made from the Sommerfeld’s atomic model.
When spectra producing atoms were placed in a magnetic or electric field
and studied using high resolving equipment, several single lines were found to
split into component lines. This phenomena lead to the idea of Orbitals
under a Subshell.
Quantum numbers describe the state and behavior of
electrons in an atom. Since electrons are governed by the
principles of quantum mechanics,
we can’t pinpoint their exact location—only probabilities.
These four quantum numbers help define the "address" or unique identity of each
electron in an atom.
The first three quantum numbers (Principal, Azimuthal and Magnetic Quantum Numbers) were obtained while solving Schrodinger's wave equation for hydrogen and other atoms.
1.
Principal
Quantum Number (n)
What
It Tells Us:
> The energy level (or shell) the electron occupies.
> The distance from
the nucleus—higher n means farther from the nucleus and higher energy.
> Defines
the size
of the orbital.
Values:
n = 1, 2, 3, 4... (Positive integers) which represent K, L, M, N, .... Shells respectively.
Effects:
As
n increases:
> Energy increases
> Orbital size increases
> Electron is less tightly bound to the
nucleus.
> Number
of electrons in corresponding shell increases by the formula 2n2.
That means when n = 3 (M Shell), the number of electron in it = 2X32
= 18.
2.
Azimuthal
Quantum Number (ℓ)
What
It Tells Us:
>
The shape
of the orbital
>
Defines the subshell
within a given principal shell
Values:
> For any value n, ℓ = 0 to (n –
1)
For example, if we wish to find the
subshells under a shell for which n = 3, then the ℓ values under it are 0 to
3-1.
i.e., ℓ = 0,
ℓ = 1,
and ℓ = 2
> The value of ℓ, its designation and shape is given in the following table.
|
ℓ |
Subshell |
Shape |
|
0 |
s |
Spherical |
|
1 |
p |
Dumbbell |
|
2 |
d |
Cloverleaf |
|
3 |
f |
Complex |
> Any shell with principal quantum number n has n number of subshells. For example the M shell (n = 3) has three subshells. They are 3s, 3p and 3d subshells.
> Determines
the angular momentum of the electron
in any subshell by the formula, mvr =
(h/2π) [ℓ(ℓ+1)]1/2
> The maximum number of electrons in a subshell
is given by the formula 2(2ℓ+1).
> Thus maximum electrons that can be
accommodated in an s – subshell (ℓ=0) = 2(2X0+1) = 2
> Thus maximum electrons that can be
accommodated in an p – subshell (ℓ=1) =6
> Thus maximum electrons that can be
accommodated in an d – subshell (ℓ=2) = 10
> Thus maximum electrons that can be accommodated in an f – subshell (ℓ=3) = 14
3.
Magnetic
Quantum Number (mₗ)
What
It Tells Us:
>
The orientation
(preferred region in space) of the orbital in 3D space
>
Distinguishes between different orbitals within the same subshell
Values:
>
For any given subshell with Azimuthal quantum number value ℓ, magnetic quantum
numbers under this subshell are given by the formula, mₗ = –ℓ to +ℓ (including 0).
>
The number of orbitals in a given shell with value ℓ is given by the formula 2ℓ+1.
> The value of ℓ, possible
mₗ
values and the number of orbitals under a given subshell is given
in the following table.
|
ℓ |
mₗ values |
Orbitals Count |
|
0 |
0 |
1 (s) |
|
1 |
–1, 0, +1 |
3 (p) |
|
2 |
–2, –1, 0, +1, +2 |
5 (d) |
|
3 |
–3, –2, –1, 0, +1, +2, +3 |
7 (f) |
> The number of orbitals in a given
shell = n2.
4.
Spin
Quantum Number (mₛ):
>This quantum number has no relation with the
solution of Schrodinger wave equation.
> German physicists Stern and Gerlach passed a
beam of neutral vapour silver atoms between the poles of a specially designed
magnet. The beam was found to split into two separate beams. It was assumed
that the unpaired electron present in silver atom is responsible for the split
of beam. Half of the silver atoms with the unpaired electrons behave like one
type of magnet and the other half behaved the opposite.
> Electron spins around its own axis along with its
orbital motion. This spin creates a small magnetic field for which an electron acts
like a tiny magnet. An electron can spin either clockwise or anticlockwise.
Accordingly there are two spin values possible for an electron while present in
an orbital.
What
It Tells Us:
> The
spin
direction of the electron
Values:
>
mₛ
= +½ (spin-up or clockwise spin or aligned with the external magnetic field)
>
mₛ
= –½ (spin-down or anticlockwise spin or aligned against the external magnetic
field)
>
Each orbital (combination of n, ℓ, mₗ) can hold 2 electrons, each
with opposite spin.
> The
spin angular momentum of an electron is given by [s(s+1)]1/2(h/2 π).
Putting the value of s=1/2 for an electron the spin angular momentum for an
electron can be calculated.
> The
z component of the spin angular momentum has two possible values. They are ½ (h/2 π)
and -1/2 (h/2 π).
Pauli’s Exclusion Principle:
Statement: No two electrons in an atom can have
all the four quantum numbers alike.
This principle has excluded the probability of finding two electrons in
an atom with similar quantum numbers.
Even we choose two electrons from the same orbital of an atom; their
Principal, Azimuthal and Magnetic quantum numbers will be same but the spin
quantum numbers will be different. This is because two electrons in an orbital
must have opposite spin; one electron with s= +1/2 and another with s = -1/2.
Application: An orbital cannot have more than two electrons. This is because the third electron
will repeat its spin quantum number with already present electrons. But this is
impossible according to Pauli’s exclusion principle.
Hund’s Rule:
Statement: The most stable electronic
arrangement in a subshell is that in which the number of unpaired electrons is
maximum. Greater the number of parallel electronic spins greater will be the
exchange energy and hence greater will be the stability.
Exchange energy: If two electrons within a subshell with parallel spin exchange their positions in space then there is a resultant decrease in energy. The energy decrease per exchange pair is called the exchange energy.
Consider p3 configuration:
We know electrons with parallel spin only can exchange their positions. From the above 3 different configurations, a. and b. configurations have two electrons with parallel spin hence in each of these cases there is only one exchange. The energy released is represented by –E. But in c. configuration there are three exchanges hence the energy released is -3E. Thus greater is the number of exchanges greater amount of energy is released and greater is the stability.
Pairing energy: When two electrons are placed in the same orbital they repeal each
other. The energy needed for pairing of electrons is called the pairing energy. Exchange energy increases the
stability whereas pairing energy decreases the stability. In the above the a.
configuration has one pair of electrons so the energy status of the electrons
is –E + P. Accordingly the energy status of p6 configuration will be -6E + 3P.
Symmetrical distribution of charge: An s orbital is spherically
symmetrical. Thus no matter it contains a single or a pair of electrons the
electronic charge is symmetrically distributed. But a p orbital is dumbell and
is symmetrical along any of the three axes. Any electronic configuration other
than a half filled or fully filled configurations is not a symmetric electronic
charge distribution where the charge is concentrated in any particular
direction. Thus if all the orbitals of a subshell are half filled or fully filled
then the electronic charge distribution is symmetrical around the nucleus which
also leads to the stability of the electronic configuration.
Hence, due to large exchange energy and symmetric distribution of charge half-filled and fully filled electronic configurations are considered to be the most stable electronic configurations.
Aufbau Principle:
The Aufbau principle is a rule used
to determine the electron configuration of atoms in their ground state.
"Aufbau" is a German word for "building up,". We
"build up" the electronic configuration by adding electrons one at a
time to the lowest-energy subshell available before moving to higher-energy subshell.
The energy content of two subshells
can be determined from the n+ℓ values. The n+ℓ value for 4s
and 3d are 4 and 5 respectively. Thus 3d is higher energetic than 4s. When n+ℓ values for two different subshells
found to be equal then the subshell with higher n value is considered higher energetic.
The n+ℓ value for 4s and 3p are 4 and
4 respectively. But 4s has higher n value. Thus 4s is higher energetic than 3p.
Applying above rule the increasing
order of subshells for filling of electrons is:
1s < 2s < 2p < 3s < 3p
< 4s < 3d …and so on…
Limitations of Hund’s rule and Aufbau principle: In general, Aufbau principle works correctly upto atomic
number 20. After that various factors are considered to decide to write the
electronic configuration. The preference of half-filled or fully filled
electronic configurations is not the only criteria for the stable electronic
configuration of an atom.
Inter electronic repulsion, screening
effect and the nucleus – electron attractive forces also contribute to the
nature of electron filling in subshells of different atoms and is confirmed
experimentally.
Electronic configuration of Cr24
= [Ar] 3d⁵ 4s¹. Here, during ionisation, the first electron is lost from 4s not
from 3d as is expected from Aufbau principle.
Image Credit:
1. Emission of energy from electron 1:
By JabberWok, CC BY-SA 3.0,
https://commons.wikimedia.org/w/index.php?curid=2639910
2. Emission of energy from electron 1:
By A_hidrogen_szinkepei.jpg: User:Szdoriderivative work:
OrangeDog (talk • contribs) - A_hidrogen_szinkepei.jpg, CC BY 2.5,
https://commons.wikimedia.org/w/index.php?curid=6273602
3. Energy level diagram as straight lines
By Rajettan - Own work, CC BY-SA 4.0,
https://commons.wikimedia.org/w/index.php?curid=41466201
4. Actual obsevation of line spectrum
By OrangeDog, CC BY-SA 3.0,
https://commons.wikimedia.org/w/index.php?curid=6278485











Hi ! Please Do Not Spam in Message and Be Honest and Respectful.